Buy US Gabapentin Online
| Gabapentin 800 mg – 180 Tabs | $189 | free | $189 | Order |
| Gabapentin 600 mg – 180 Tabs | $179 | free | $179 | Order |
| Gabapentin 400 mg – 180 Tabs | $169 | free | $169 | Order |
| Gabapentin 300 mg – 180 Tabs | $159 | free | $159 | Order |
| Generic Fioricet 325/50/40mg – 180 Tabs | $239 | free | $239 | Order |
| Generic Fioricet 325/50/40mg – 120 Tabs | $199 | free | $199 | Order |
| Generic Fioricet 325/50/40mg – 90 Tabs | $169 | free | $169 | Order |
We guarantee the best fioricet, Gabapentin, and generic fioricet, butalbital apap caffeine online at the cheapest prices. We provide free delivery using USPS priority mail delivery service by COD payment services.

All Pharmacies associated are licensed to distribute in the states, you can be 100% sure to receive the same quality medication that you get from your local drug store. Upon receiving a valid prescription of the product you buy. Our US licensed pharmacies will fill a prescription for a medication that is FDA approved. To assure confidentiality and privacy our US licensed Pharmacy will fill and ship your prescription in a discreet package.
We have several pharms to place your orders. They are all us licensed pharmacies and never sell controlled substances. We will send you order ID and tracking ID together within two business days after you placed your orders.
But It is beyond our control after I sent you order ID and tracking ID. Please ignore any call from call centers because we never call customers to refill orders.
You do not need contact us for your tracking ID because we will send you tracking ID as soon as we get it.
By US law, returned packages are no use any more. We have to pay the pharmacies to cover the lose. It is very hard to do online pharm business. We saved both your and doctors’ time to set up the online pharmacy websites.
Neurontin (gabapentin)
| Commonly used brand name(s): |
| In the U.S. – |
|
| Available Dosage Forms: |
|
Gabapentin Description
Neurontin is a prescription drug for treating symptoms of epilepsy. It works as an anticonvulsant. It is the brand name for gabapentin. This medicine works on the nerves in the body, which are responsible for causing seizures. Besides working as an anticonvulsant, this drug is also used to relieve pain as the medicine has a calming effect on the nerves. This drug is mostly used as a pain reliever for pain caused in the nerves due to shingles and herpes. Neurontin is manufactured by Parke-Davis.
Doctors usually prescribe this drug alone or in combination with other drugs. This drug is not prescribed for children below the age of 12 years. Even if it is prescribed to children between 3 to 12 years, it is mostly given to children with a combination of other drugs. The drug is usually available in two forms- in tablets and in the liquid form. Make sure that the tablets are stored at room temperature, away from light and moisture and outside the reach of children. If you are using the liquid form of this drug, you can keep the bottle in the refrigerator but make sure that you do not freeze the liquid medicine.
Gabapentin is used to help control partial seizures (convulsions) in the treatment of epilepsy. This medicine cannot cure epilepsy and will only work to control seizures for as long as you continue to take it.
Gabapentin is also used in adults to manage a condition called postherpetic neuralgia, which is pain that occurs after “shingles.”
Gabapentin extended-release tablets is used to treat a condition called Restless Legs Syndrome (RLS). RLS is a neurologic disorder that affects sensation and movement in the legs to feel uncomfortable. This results in an irresistible feeling of wanting to move your legs to make them comfortable.
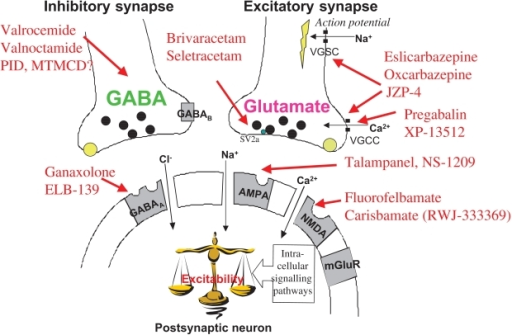
Gabapentin is an anticonvulsant. It increases the amount of a chemical called gamma-aminobutyric acid (GABA) in the brain. It is felt that some epileptic seizures occur when there are low levels of GABA in the brain. By increasing the amount of GABA, gabapentin reduces the number of seizures.
Gabapentin also works to relieve pain for certain conditions in the nervous system. It is not used for routine pain caused by minor injuries or arthritis.
Conditions treated by Gabapentin
The main use of this medicine is to treat symptoms of epilepsy as it works as an anticonvulsant and is effective in controlling seizures. Besides this, Neurontin is also used as a pain reliever for nerve pains and pain caused due to migraine as this drug has a soothing effect on nerves. The drug is used to treat pain caused by abnormal neurological functioning or pain caused due to injury or damage of nerves. Some doctors also use this drug to treat anxiety disorders. It has been seen that this drug has been successful in treating symptoms of anxiety disorders like panic attacks, tension, hypertension, high rate of heartbeat, chest pain, difficulty in breathing, post traumatic stress disorder, etc.
By BuyGabapentin800mg.com, Gabapentin Can be used for a lot of Nerve Pain related health conditions including Cough, Hot Flashes, Alcohol Withdrawal, Anxiety 161 reviews, Bipolar Disorder, Trigeminal Neuralgia, Postherpetic Neuralgia, Migraine, Insomnia? Occipital Neuralgia? Peripheral Neuropathy?Vulvodynia, Benign Essential Tremor, Epilepsy, Fibromyalgia, Pain Relief, Diabetic Peripheral Neuropathy , Neuropathic Pain?Reflex Sympathetic Dystrophy Syndrome?Periodic Limb Movement Disorder? Spondylolisthesis? Burning Mouth Syndrome?Pudendal Neuralgia? Small Fiber Neuropathy.
The mechanism of action of gabapentin in neuropathic pain.
Neuropathic pain is a common and potentially treatable cause of considerable lifelong morbidity. Effective pharmacological treatments are scarce, but one group of drugs that has shown promise is the antiepileptics.
Gabapentin has become popular as a first-line treatment for neuropathic pain because of its efficacy as an antineuropathic agent and relatively benign side-effect profile. However, its mechanism of action is far from clear.
This review discusses the available evidence for the postulated mechanisms of action of gabapentin. Understanding the mechanism of action of this agent may well lead to the development of safer and more effective antineuropathic drugs.
Gabapentin Dosage information
a.) Typical Dosage Recommendations
The basic dose of Neurontin is 300 mg. This drug should be taken as per the doctor’s recommendations. Do not take in larger amounts of this medicine as it could cause serious side effects later on. Do not prolong the use of this medicine without consulting your doctor. It could cause damage to your nerves. The drug can be taken with or without food. The medicine comes in the form of tablets. If you have broken a tablet, make sure that you take in the broken tablet as soon as possible, possibly as your next dose. If you are using the liquid form of the medicine, always make sure that you are measuring the liquid in a proper measuring cup and not in spoons. Measuring in spoons will not give you the proper measurement of the medicine and that will interfere with the dose that you are supposed to have.
b.) Missing a Dose
In case you have missed a dose of Neurontin, take the missed dose as soon as you remember about it. However, if the time of the next dose is close by, it is wise to wait for the next dose and not have the missed dose. Having two doses of the medicine in a very short time span will cause overdosing which may lead to side effects.
c.) Overdosing
Get in touch with your doctor as soon as you realize that you have had extra doses of the medicine. You will need immediate medical attention in the event of overdoing of this drug. There can be side effects of overdosing like drowsiness, slurred speech, blurred vision, weakness, diarrhea, etc.
Gabapentin Warnings
Make sure you tell your doctor about your complete medical history when he/she is prescribing this drug to you. This drug might not be suitable for you if you are suffering from kidney, liver or heart diseases. You should also inform your doctor if you have an allergic reaction to gabapentin. Avoid having alcohol while you are on this medicine. Antacids can interfere with the absorption of Neurontin; therefore, it is advisable not to have Antacids at least 2 hours before having this medicine. In case you are pregnant or are planning to become pregnant, do not forget to inform the same to your doctor. Doctors do not generally prescribe this drug to pregnant ladies unless there is an immediate and genuine need for it.
This medicine is not meant for children below the age of 12 years. Many people experience side effects like blurred vision and drowsiness after taking this drug. Therefore, it is advisable not to drive after taking the medicine. Allow your body to get adapted to this new drug. Visit the doctor frequently as he/she will want to carry out regular tests to see how your body is adapting to this drug.
Gabapentin Side effects
-
-
- Allergic reactions
- swollen glands
- hives
- swelling up of face, lips, throat or tongue
- fever
- Changes in behavior
- Mood swing
- anxiety
- depression
- hyperactive
- having suicidal thoughts, restlessness, and agitation.
- Symptoms of flu- Fever, body aches, chills
- Increased seizures
- Tremors
- Getting bruised easily
- Fast back and forth movement of eyes
- Blurred vision
- Lack of coordination
- Weakness
- drowsiness
- extreme tiredness
-
There are some side effects that may occur among children. Here are some of them:
- Problem in concentrating
- Memory problems
- Sudden changes in behavior
Gabapentin Mechanism of action
The mechanism of the anticonvulsant action of gabapentin has not been fully described. Several possible mechanisms for pain improvement have been discussed. Though similar in structure to the endogenous neurotransmitter GABA, gabapentin has not been shown to bind to GABA receptors at concentrations at or below 1 mM. Gabapentin modulates the action of glutamate decarboxylase (GAD) and branched chain aminotransferase (BCAT), two enzymes involved in GABA biosynthesis. In human and rat studies, gabapentin was found to increase GABA biosynthesis, and to increase non-synaptic GABA neurotransmission in vitro.
Gabapentin prevents seizures in a wide variety of models in animals, including generalized tonic-clonic and partial seizures. Gabapentin has no activity at GABAA or GABAB receptors of GABA uptake carriers of brain. Gabapentin interacts with a high-affinity binding site in brain membranes, which has recently been identified as an auxiliary subunit of voltage-sensitive Ca2+ channels.
Gabapentin has been shown to bind to the α2δ-1 subunit of voltage gated calcium ion channels, which contributes to its pain attenuation effects in diabetic neuropathy and post-herpetic neuralgia. Other neurophysiological findings indicate that gabapentin also interacts with NMDA receptors, protein kinase C, and inflammatory cytokines.
Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Gabapentin is also active in models that detect anxiolytic activity.
Although gabapentin may have several different pharmacological actions, it appears that modulation of GABA synthesis and glutamate synthesis may be important.
Gabapentin interacts with cortical neurons at auxillary subunits of voltage-sensitive calcium channels. Gabapentin increases the synaptic concentration of GABA, enhances GABA responses at non-synaptic sites in neuronal tissues, and reduces the release of mono-amine neurotransmitters.
One of the mechanisms implicated in this effect of gabapentin is the reduction of the axon excitability measured as an amplitude change of the presynaptic fibre volley (FV) in the CA1 area of the hippocampus. This is mediated through its binding to presynaptic NMDA receptors.
Other studies have shown that the antihyperalgesic and antiallodynic effects of gabapentin are mediated by the descending noradrenergic system, resulting in the activation of spinal alpha2-adrenergic receptors. Gabapentin has also been shown to bind and activate the adenosine A1 receptor.
Possible Drug interactions with Gabapentin
There are several drugs that may react with Neurontin if they are taken together. Make sure that you tell your doctor clearly about your medical history.
Specify what other drugs you are already taking if you have been prescribed Neurontin by your doctor. This includes all types of multi vitamin medicines, calcium or iron supplements, herbal supplements or any other type of over-the-counter medicines.
Antacids react greatly with Neurontin. Therefore, do not take in an Antacid at least 2 hours before having Neurontin. This drug also reacts with:
-
-
- hydrocodone
- morphine
- Naprosyn(naproxen)
-
Therefore, inform your doctor if you take medicines which might be containing any of these active ingredients.
If you have any questions about buying discount Neurontin online or any other prescription products you can contact our team of professional Patient Service Representatives or one of our pharmacists 24-7 by calling 1-800-226-3784.
Notice: The above information is an educational aid only. It is not intended as medical advice for individual conditions or treatments. Talk to your doctor, nurse or pharmacist before following any medical regimen to see if it is safe and effective for you.
Gabapentin Memory Loss Side Effects
Just like many other medication. The gabapentin may produce allergic reaction to the patients. As the symptoms for allergies vary from person to person. Always contact your doctor if you present any kind of side effect.
There are rumors that the gabapentin causes memory loss as one of the side effects. While it is still unclear that you actually forget about recent events. It has been proven that you might present some memory problems, suffer from headaches and have trouble concentrating or reacting.
This is only the mental part of the side effects of the gabapentin related to the memory loss.There are also emotional changes that you might experience as well as a variety of different physical diseases.
When you have been prescribed with this or any other strong medication by your doctor.Always pay attention to any of the possible side effects. If you feel that anything is going wrong, contact your doctor immediately for attention. It might be normal but it also might prevent something serious.
Who is not suitable for Ordering Gabapentin Online ?
Normally Gabapentin is suitable for all adult and children bigger than six years old. But you are not allowed to order Gabapentin online if you have any of following health conditions (But you are OK to order in your local street pharmacies):
-
-
- You are younger than 18 years old;
- You have kidney disease;
- diabetes;
- liver disease and heart diseases;
- a history of depression, mood disorder, drug abuse, or suicidal thoughts or actions;
- (for patients with RLS) if you are a day sleeper or work a night shift;
- You are breastfeeding mother or you are pregnant;
- have thoughts about suicide.
-
Stop immediately if you have any thoughts about suicide. Donot order Gabapentin online if you have suicide thoughts. Please go to your doctor to have your completely checked.
Gabapentin for Central Nervous System Depression
RESPIRATORY DEPRESSION
Gabapentin has been associated with central nervous system (CNS) depression including sedation, somnolence, loss of consciousness as well as serious cases of respiratory depression. Patients with compromised respiratory function, respiratory or neurological disease, renal impairment and the elderly are at higher risk of experiencing these severe adverse effects. Concomitant use of CNS depressants with gabapentin is also a contributing factor.
CONCOMITANT USE WITH OPIOIDS
Concomitant use of opioids with NEURONTIN potentiates the risk of respiratory depression, profound sedation, syncope, and death. Gabapentin concentrations may also increase in patients receiving concomitant opioid (See DRUG INTERACTIONS).
Patients who require concurrent treatment with opioids or other CNS depressants should be observed carefully for signs and symptoms of CNS depression, and the dose of gabapentin or opioid should be reduced accordingly. See also DOSAGE AND ADMINISTRATION, Dosing Considerations.
Carcinogenesis and Mutagenesis
Gabapentin produced an increased incidence of acinar cell adenomas and carcinomas in the pancreas of male rats, but not female rats or in mice, in oncogenic studies with doses of 2000 mg/kg which resulted in plasma concentrations 14 times higher than those occurring in humans at a dose of 2400 mg/day. The relevance of these pancreatic acinar cell tumours in male rats to humans is unknown, particularly since tumours of ductal rather than acinar cell origin are the predominant form of human pancreatic cancer. (See TOXICOLOGY, Carcinogenicity Studies).
Dependence/Tolerance
The abuse and dependence potential of gabapentin has not been evaluated in human studies. Cases of abuse and dependence have been reported in the post-marketing database. These individuals were taking higher than recommended doses of gabapentin for unapproved uses. Most of the individuals described in these reports had a history of polysubstance abuse or used gabapentin to relieve symptoms of withdrawal from other substances. As with any CNS active drug, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of abuse or misuse of Neurontin (e.g. development of tolerance, self-dose escalation, and drug-seeking behavior).
There are rare post-marketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not indicated. Such symptoms included agitation, disorientation and confusion after suddenly discontinuing gabapentin that resolved after restarting gabapentin. Most of these individuals had a history of poly-substance abuse or used gabapentin to relieve symptoms of withdrawal from other substances.

Hypersensitivity
SERIOUS DERMATOLOGICAL REACTIONS
There have been post-marketing reports of Stevens-Johnson syndrome (SJS) and Erythema multiforme (EM) in patients during treatment with gabapentin. Should signs and symptoms suggest SJS or ER, gabapentin should be discontinued immediately. (see Post-Marketing Adverse Drug Reactions)
There have been reports in the post-marketing experience of hypersensitivity including systemic reactions and cases of urticaria and angioedema. (see Post-Marketing Adverse Drug Reactions)
DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS (DRESS)
Severe, life-threatening, systemic hypersensitivity reactions such as Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome have been reported in patients taking antiepileptic drugs including gabapentin.
It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Gabapentin should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Prior to initiation of treatment with gabapentin, the patient should be instructed that a rash or other signs or symptoms of hypersensitivity such as fever or lymphadenopathy may herald a serious medical event and that the patient should report any such occurrence to a physician immediately.
ANAPHYLAXIS
Gabapentin can cause anaphylaxis. Signs and symptoms in reported cases have included difficulty breathing, swelling of the lips, throat and tongue and hypotension requiring emergency treatment. Patients should be instructed to discontinue gabapentin and seek immediate medical care should they experience signs or symptoms of anaphylaxis.
Neurologic
Gabapentin treatment has been associated with dizziness and somnolence, which could increase the occurrence of accidental injury (fall). There have also been postmarketing reports of agitation, confusion, loss of consciousness and mental impairment. Therefore, patients should be advised to exercise caution until they are familiar with the potential effects of the medication. (See DOSAGE AND ADMINISTRATION, Dosing Considerations and Special Patient Populations).
Psychiatric
SUICIDAL IDEATION AND BEHAVIOUR
Suicidal ideation and behaviour have been reported in patients treated with antiepileptic agents in several indications.
All patients treated with antiepileptic drugs, irrespective of indication, should be monitored for signs of suicidal ideation and behaviour and appropriate treatment should be considered. Patients (and caregivers of patients) should be advised to seek medical advice should signs of suicidal ideation or behaviour emerge.
An FDA meta-analysis of randomized placebo controlled trials, in which antiepileptic drugs were used for various indications, has shown a small increased risk of suicidal ideation and behaviour in patients treated with these drugs. The mechanism of this risk is not known.
There were 43,892 patients treated in the placebo controlled clinical trials that were included in the meta-analysis. Approximately 75% of patients in these clinical trials were treated for indications other than epilepsy and, for the majority of non-epilepsy indications the treatment (antiepileptic drug or placebo) was administered as monotherapy. Patients with epilepsy represented approximately 25% of the total number of patients treated in the placebo controlled clinical trials and, for the majority of epilepsy patients, treatment (antiepileptic drug or placebo) was administered as adjunct to other antiepileptic agents (i.e., patients in both treatment arms were being treated with one or more antiepileptic drug). Therefore, the small increased risk of suicidal ideation and behaviour reported from the meta-analysis (0.43% for patients on antiepileptic drugs compared to 0.24% for patients on placebo) is based largely on patients that received monotherapy treatment (antiepileptic drug or placebo) for non-epilepsy indications. The study design does not allow an estimation of the risk of suicidal ideation and behaviour for patients with epilepsy that are taking antiepileptic drugs, due both to this population being the minority in the study, and the drug-placebo comparison in this population being confounded by the presence of adjunct antiepileptic drug treatment in both arms.
Special Populations
Pregnant Women: Based on animal data, gabapentin may cause fetal harm (see TOXICOLOGY – Reproduction Studies). In non-clinical studies in mice, rats and rabbits, gabapentin was developmentally toxic (e.g., increased fetal skeletal and visceral abnormalities, and increased embryofetal mortality) when administered to pregnant animals at doses lower than the maximum recommended human dose (MRHD) of 3600 mg/day on a body surface area (mg/m2) basis.
Teratogenic Potential: Gabapentin crosses the human placental barrier. Although there are no adequate and well-controlled studies in pregnant women, congenital malformations and adverse pregnancy outcomes have been reported with gabapentin use, both from literature and Pregnancy Registries. Since the potential risk for humans is uncertain, gabapentin should only be used during pregnancy if the potential benefit to the mother outweighs the potential risk to the fetus. If women decide to become pregnant while taking NEURONTIN, the use of this product should be carefully re-evaluated.
Pregnancy Registry: Physicians are advised to recommend that pregnant patients taking NEURONTIN enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the following website: http://www.aedpregnancyregistry.org/.
Nursing Women: Gabapentin is excreted in human milk. There are no controlled studies on the effects of gabapentin on breast-fed infants. Because of the potential for serious adverse reactions in nursing infants, a decision should be made as to whether to discontinue nursing or to discontinue NEURONTIN, taking into account the benefit of the drug to the mother.
Pediatrics: The safety and efficacy in patients under the age of 18 have not been established.
Safety data in 39 patients between the ages of 12 and 18 years included in the double-blind, placebo-controlled trials showed that, at doses of 900 to 1200 mg/day, the incidence of adverse events in this group of patients was similar to that observed in older individuals.
In controlled clinical trials involving patients, 3 to 12 years of age (N=323), psychiatric adverse events such as emotional lability, hostility, hyperkinesia and thought disorder were reported at a higher frequency in patients treated with gabapentin compared to placebo.
Geriatrics: Systematic studies in geriatric patients have not been conducted. Adverse clinical events reported among 59 patients over the age of 65 years treated with Neurontin did not differ from those reported for younger individuals. The small number of individuals evaluated and the limited duration of exposure limits the strength of any conclusions reached about the influence of age, if any, on the kind and incidence of adverse events associated with the use of Neurontin.
As Neurontin is eliminated primarily by renal excretion, dosage adjustment may be required in elderly patients because of declining renal function. (See DOSAGE AND ADMINISTRATION, Dosing Considerations; ACTION AND CLINICAL PHARMACOLOGY, Special Populations and Conditions).
Monitoring and Laboratory Tests
Clinical trials data do not indicate that routine monitoring of clinical laboratory parameters is necessary for the safe use of Neurontin. Neurontin may be used in combination with other commonly used antiepileptic drugs without concern for alteration of the blood concentrations of gabapentin or other antiepileptic drugs.
How should this medicine be used?
Gabapentin comes as a capsule, a tablet, an extended-release (long-acting) tablet, and an oral solution (liquid) to take by mouth. Gabapentin capsules, tablets, and oral solution are usually taken with a full glass of water (8 ounces [240 milliliters]), with or without food, three times a day.
These medications should be taken at evenly spaced times throughout the day and night; no more than 12 hours should pass between doses. The extended-release tablet (Horizant) is taken with food once daily at about 5 PM. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take gabapentin exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
Gabapentin extended-release tablets cannot be substituted for another type of gabapentin product. Be sure that you receive only the type of gabapentin that was prescribed by your doctor. Ask your pharmacist if you have any questions about the type of gabapentin you were given.
Swallow the extended-release tablets whole; do not cut, chew, or crush them.
If your doctor tells you to take one-half of a regular tablet as part of your dose, carefully split the tablet along the score mark. Use the other half-tablet as part of your next dose. Properly throw away any half-tablets that you have not used within several days of breaking them.
If you are taking gabapentin to control seizures or PHN, your doctor will probably start you on a low dose of gabapentin and gradually increase your dose as needed to treat your condition. If you are taking gabapentin to treat PHN, tell your doctor if your symptoms do not improve during your treatment.
Gabapentin may help to control your condition but will not cure it. Continue to take gabapentin even if you feel well. Do not stop taking gabapentin without talking to your doctor, even if you experience side effects such as unusual changes in behavior or mood. If you suddenly stop taking gabapentin tablets, capsules, or oral solution, you may experience withdrawal symptoms such as anxiety, difficulty falling asleep or staying asleep, nausea, pain, and sweating. If you are taking gabapentin to treat seizures and you suddenly stop taking the medication, you may experience seizures more often. Your doctor may decrease your dose gradually over at least a week.
Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide) when you begin treatment with gabapentin and each time you refill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (http://www.fda.gov/Drugs) or the manufacturer’s website to obtain the Medication Guide.
Other uses for this medicine
Gabapentin is also sometimes used to relieve the pain of diabetic neuropathy (numbness or tingling due to nerve damage in people who have diabetes), and to treat and prevent hot flashes (sudden strong feelings of heat and sweating) in women who are being treated for breast cancer or who have experienced menopause (”change of life”, the end of monthly menstrual periods). Talk to your doctor about the risks of using this medication for your condition.
How Gabapentin is typically packaged?
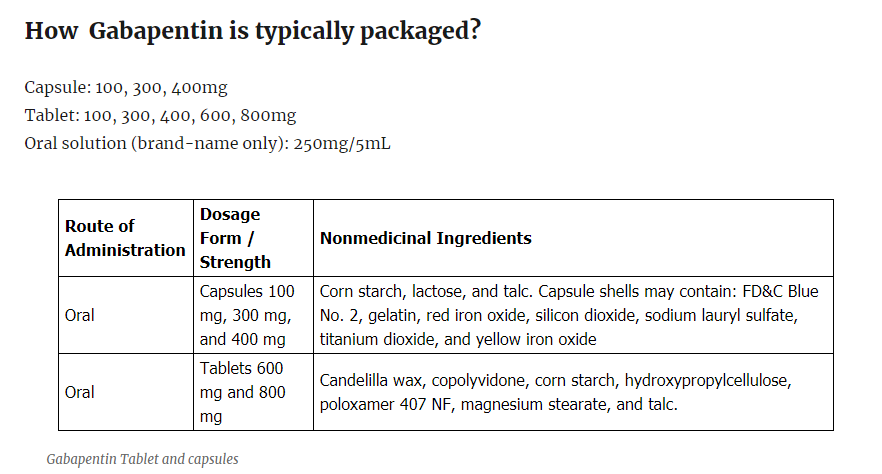
Capsule: 100, 300, 400mg
Tablet: 100, 300, 400, 600, 800mg
Oral solution (brand-name only): 250mg/5mL
Normal Dosage Of Gabapentin
USAGE FOR EPILEPSY: The usual starting dose is 300mg for adults and children over 12 years of age. This dose is taken on the evening of the first day. Your doctor may then increase the dose on the second day to 300mg in the morning, and 300mg in the evening. On the third day you may be increased to 300mg three times per day. The maximum dosage is 3600mg per day. Gabapentin is not recommended for children under 12 years.
USAGE FOR DIABETIC NERVE PAIN: The dosage follows the rules for epilepsy, and may be continued for up to 5 months. If you suffer from any kidney problems, the doctor may issue a lower dosage.
USAGE AS A NERVE BLOCK: Gabapentin is not marketed for use as a nerve block, but is commonly used as such following successful applications. The dosage follows the same rules as for epilepsy patients, with a maximum dosage of 3600mg. For chronic pain conditions, Gabapentin may need
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.
What special precautions should I follow?
Before taking gabapentin,
-
-
- tell your doctor and pharmacist if you are allergic to gabapentin, any other medications, or any of the inactive ingredients in the type of gabapentin you plan to take. Ask your pharmacist for a list of the inactive ingredients.
- you should know that gabapentin is available in different forms that may be prescribed for different uses. Ask your doctor to be sure that you are not taking more than one product that contains gabapentin.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: hydrocodone (in Hydrocet, in Vicodin, others), medications that make you feel dizzy or drowsy, morphine (Avinza, Kadian, MSIR, others), and naproxen (Aleve, Anaprox, Naprosyn, others). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- if you are taking antacids such as Maalox or Mylanta, take them at least 2 hours before you take gabapentin tablets, capsules, or solution.
- tell your doctor if you have or have ever had kidney disease. If you will be taking the extended-release tablets, also tell your doctor if you need to sleep during the day and stay awake at night.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking gabapentin, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking gabapentin.
- you should know that this medication may make you drowsy or dizzy, may slow your thinking, and may cause loss of coordination. Do not drive a car or operate machinery until you know how this medication affects you, and your doctor agrees that it is safe for you to begin these activities.
- if you are giving gabapentin to your child, you should know that your child’s behavior and mental abilities may change while he or she is taking gabapentin. Your child may have sudden changes in mood, become hostile or hyperactive, have difficulty concentrating or paying attention, or be drowsy or clumsy. Have your child avoid activities that could be dangerous, such as riding a bicycle, until you know how gabapentin affects him or her.
- remember that alcohol can add to the drowsiness caused by this medication.
- you should know that your mental health may change in unexpected ways and you may become suicidal (thinking about harming or killing yourself or planning or trying to do so) while you are taking gabapentin for the treatment of epilepsy, mental illness, or other conditions. A small number of adults and children 5 years of age and older (about 1 in 500 people) who took anticonvulsants such as gabapentin to treat various conditions during clinical studies became suicidal during their treatment. Some of these people developed suicidal thoughts and behavior as early as one week after they started taking the medication. There is a risk that you may experience changes in your mental health if you take an anticonvulsant medication such as gabapentin, but there may also be a risk that you will experience changes in your mental health if your condition is not treated. You and your doctor will decide whether the risks of taking an anticonvulsant medication are greater than the risks of not taking the medication. You, your family, or your caregiver should call your doctor right away if you experience any of the following symptoms: panic attacks; agitation or restlessness; new or worsening irritability, anxiety, or depression; acting on dangerous impulses; difficulty falling or staying asleep; aggressive, angry, or violent behavior; mania (frenzied, abnormally excited mood); talking or thinking about wanting to hurt yourself or end your life; withdrawing from friends and family; preoccupation with death and dying; giving away prized possessions; or any other unusual changes in behavior or mood. Be sure that your family or caregiver knows which symptoms may be serious so they can call the doctor if you are unable to seek treatment on your own.
-
What special dietary instructions should I follow?
Unless your doctor tells you otherwise, continue your normal diet.
What should I do if I forget a dose?
If you forget to take gabapentin capsules, tablets, or oral solution, take the missed dose as soon as you remember it. However, if it is almost time for the next dose or if you forget to take gabapentin extended-release tablets, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
What side effects can this medication cause?
Gabapentin may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
drowsiness
-
-
- tiredness or weakness
- dizziness
- headache
- uncontrollable shaking of a part of your body
- double or blurred vision
- unsteadiness
- anxiety
- memory problems
- strange or unusual thoughts
- unwanted eye movements
- nausea
- vomiting
- heartburn
- diarrhea
- dry mouth
- constipation
- increased appetite
- weight gain
- swelling of the hands, feet, ankles, or lower legs
- back or joint pain
- fever
- runny nose, sneezing, cough, sore throat, or flu-like symptoms
- ear pain
- red, itchy eyes (sometimes with swelling or discharge)
-
Some side effects may be serious. If you experience any of the following symptoms, call your doctor immediately:
-
-
- rash
- itching
- swelling of the face, throat, tongue, lips, or eyes
- hoarseness
- difficulty swallowing or breathing
- seizures
-
Gabapentin may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration’s (FDA) MedWatch Adverse Event Reporting program online [at http://www.fda.gov/Safety/MedWatch] or by phone [1-800-332-1088].
What should I know about storage and disposal of this medication?
Keep this medication in the container it came in, tightly closed, and out of reach of children. Store the tablets, extended-release tablets, and capsules at room temperature, away from excess heat and moisture (not in the bathroom). Store the oral solution in the refrigerator. Throw away any medication that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medication.
In case of emergency/overdose
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
Symptoms of overdose may include the following:
- double vision
- slurred speech
- drowsiness
- diarrhea
- Buy Gabapentin (Neurontin) without Prescription
- How does Gabapentin Work ?
- Gabapentin, impotence and other problems?
- Gabapentin Drug Interaction
- Gabapentin is used for Restless legs syndrome
- Gabapentin is used for Neuropathic pain (other than postherpetic neuralgia)
- Gabapentin Usage for Alcohol disorder and Alcohol withdrawal
- Gabapentin Pharmacology
- Gabapentin Side Effects For Healthcare Professionals
- Gabapentin Side Effects
- Gabapentin Breastfeeding Warnings
- Gabapentin Pregnancy Warnings
- Protect yourself from scams, buy Fioricet safely online.
- Types of migraines
- Migraine – Causes and Cure
What other information should I know?
Gabapentin Drug-Drug Interactions
The drug interaction data described in this subsection were obtained from studies involving healthy adults and adult patients with epilepsy:
Antiepileptic Agents
There is no interaction between Neurontin (gabapentin) and phenytoin, valproic acid, carbamazepine, or phenobarbital. Consequently, Neurontin may be used in combination with other commonly used antiepileptic drugs without concern for alteration of the plasma concentrations of gabapentin or the other antiepileptic drugs.
Hydrocodone
Co-administration of single doses of gabapentin (125 mg to 500 mg; N= 48) and hydrocodone (10 mg; N= 50) decreased the Cmax and AUC values of hydrocodone in a dose-dependent manner relative to administration of hydrocodone alone. The Cmax and AUC values for hydrocodone were 2% and 4% lower, respectively, after administration of 125 mg gabapentin and 16% and 22% lower, respectively, after administration of 500 mg gabapentin. The mechanism for this interaction is unknown. Hydrocodone increased gabapentin AUC values by 14%. The magnitude of interaction with higher doses of gabapentin is not known.
Morphine
A literature article reported that when a 60 mg controlled release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule in healthy volunteers (N= 12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Morphine pharmacokinetic parameter values were not affected by administration of gabapentin 2 hours after morphine in this study. Because this was a single dose study, the magnitude of the interaction at steady state and at higher doses of gabapentin are not known.
Naproxen
In healthy adult volunteers (N= 18), the co-administration of single doses of naproxen sodium capsules (250 mg) and gabapentin (125 mg) increased the amount of gabapentin absorbed by 12% to 15%. Gabapentin did not affect naproxen pharmacokinetic parameters in this study. These doses are lower than the therapeutic doses for both drugs. Therefore, the magnitude of interaction at steady state and within the recommended dose ranges of either drug is not known.
Oral Contraceptives
Coadministration of gabapentin with the oral contraceptive Norlestrin® does not influence the steady-state pharmacokinetics of norethindrone or ethinyl estradiol.
Antacids
Coadministration of gabapentin with an aluminum and magnesium-based antacid reduces gabapentin bioavailability by up to 20%. Although the clinical significance of this decrease is not known, co-administration of similar antacids and gabapentin is not recommended.
Cimetidine
A slight decrease in renal excretion of gabapentin observed when it is coadministered with cimetidine is not expected to be of clinical importance. The effect of gabapentin on cimetidine has not been evaluated.
Probenecid
Renal excretion of gabapentin is unaltered by probenecid.
Keep all appointments with your doctor.
Before having any laboratory test, tell your doctor and the laboratory personnel that you are taking gabapentin.
If you use a dipstick to test your urine for protein, ask your doctor which product you should use while taking this medication.
Do not let anyone else take your medication. Ask your pharmacist any questions you have about refilling your prescription.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.